What is a KKDIK Only Representative?
A KKDIK Only Representative is a natural or legal person established in Türkiye, appointed by a foreign manufacturer, formulator, or article producer not established in Türkiye. The OR fulfils all importer obligations under the KKDIK Regulation, enabling foreign companies to place substances, mixtures, or articles on the Turkish market.
Through Chemleg’s KKDIK Only Representative services, companies gain direct access to the Turkish market while ensuring full regulatory compliance.
When is an Only Representative Required?
According to Article 9 of the KKDIK Regulation (Turkey REACH):
- A non-Turkish manufacturer, formulator, or article producer placing substances, mixtures, or articles on the Turkish market may appoint a natural or legal person established in Türkiye as an Only Representative (OR).
- Once appointed, the OR fully assumes the importer obligations under KKDIK, including registration and compliance responsibilities.
- The OR must maintain up-to-date records on imported volumes, customers, and the latest version of the Safety Data Sheet (SDS).
- Importers covered by the OR are no longer considered importers under KKDIK; instead, they are classified as downstream users.
For your company: Appointing an OR ensures full regulatory compliance, protects your Turkish partners from unnecessary obligations, and secures uninterrupted access to the Turkish market.
Why Appoint an Only Representative?
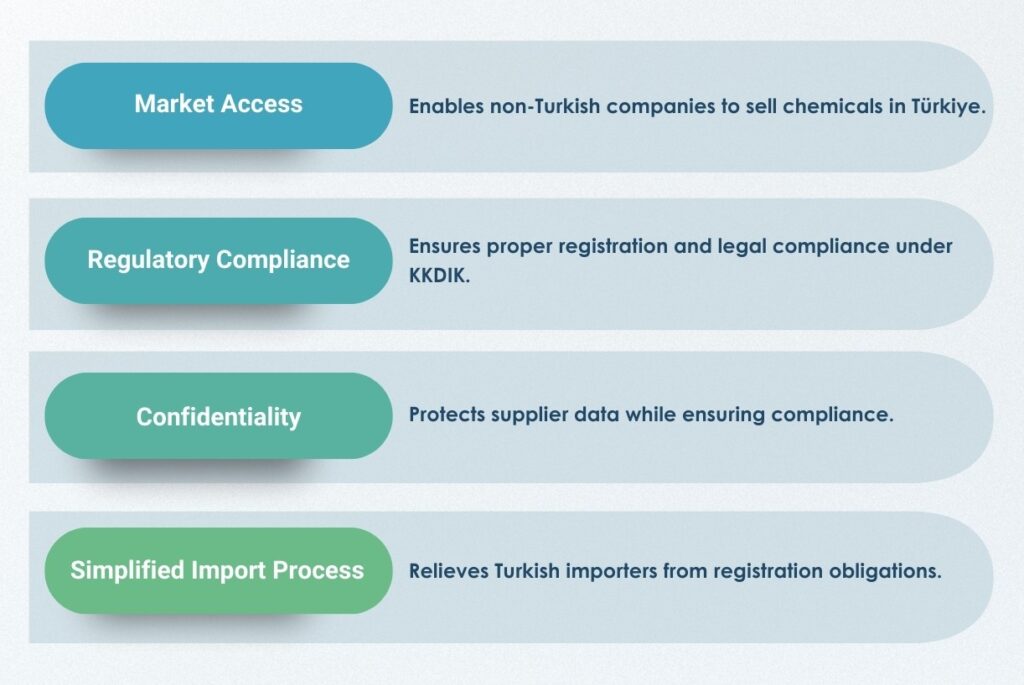
By completing registration and compliance processes in line with the KKDIK Regulation, the Only Representative ensures that companies can place their products on the Turkish market, while also relieving Turkish importers of the compliance burden.
What to Consider When Selecting an Only Representative?
- Expertise in KKDIK: Proven knowledge of chemical regulation and dossier preparation.
- Qualified Professionals: Chemists, chemical engineers, and regulatory experts on staff.
- Infrastructure: Access to IUCLID, KKS, and tools for CSR/SDS preparation.
- Transparency: Clear communication, proactive risk management, and practical solutions.
Chemleg has successfully acted as KKDIK Only Representative for numerous companies, ensuring safe and compliant market presence in Türkiye.
What Services Does Chemleg Provide as an Only Representative?
- Substance Registration & Dossier Preparation – Managing IUCLID dossiers and KKS (Turkish Chemical Registration System) submissions
- Chemical Safety Report (CSR) & Risk Assessment – Ensuring hazard evaluations are compliant
- Supply Chain Communication – Keeping importers informed about compliance status
- SIEF & Consortium Participation – Managing data-sharing obligations
- Post-Registration Compliance – Handling updates, amendments, and new obligations
Fill in the form now to schedule a consultation with our experts.
Frequently Asked Questions
What are the main responsibilities of a KKDIK Only Representative?
A KKDIK OR completes pre-registration and registration, manages communication with the Ministry, collects information from the manufacturer, and informs Turkish importers.
For your company: This means a single trusted partner handling all regulatory obligations on your behalf.
What happens if no Only Representative is appointed?
Without an OR, the Turkish importer must register the chemicals under KKDIK. This is costly, complex, and non-compliance can lead to fines or market bans.
For your company: Appointing an OR removes this burden from your Turkish partners and secures smoother market entry.
Does the OR take over all importer obligations?
Yes. The OR fully assumes the importer’s role, including registration, notifications, updates, and regulatory correspondence. Importers covered by the OR are regarded as downstream users.
Can more than one OR be appointed?
No. Under KKDIK, only one OR can be appointed per manufacturer for the same substance. Overlapping appointments are invalid.







