What is risk assessment of biocidal products?
Risk assessment of biocidal products is the process carried out to ensure that these products do not pose an unacceptable risk to human health, animal health, or the environment. This assessment is based on the active substances and substances of concern of the biocidal products, exposure scenarios, and intended uses.
Under the Biocidal Products Regulation (BPR), it is a mandatory step for the authorisation of biocidal products intended for placement on the EU market. Chemleg helps you successfully complete this step to ensure regulatory compliance and market access.
Who Needs a Biocidal Product Risk Assessment?
Those responsible for conducting biocidal product risk assessments include:
• Manufacturers of Biocidal Active Substances & Formulations
- Importers & Representatives of Biocidal Products
- Producers of Biocidal-Treated Articles
Chemleg’s Biocidal Product Risk Assessment Services
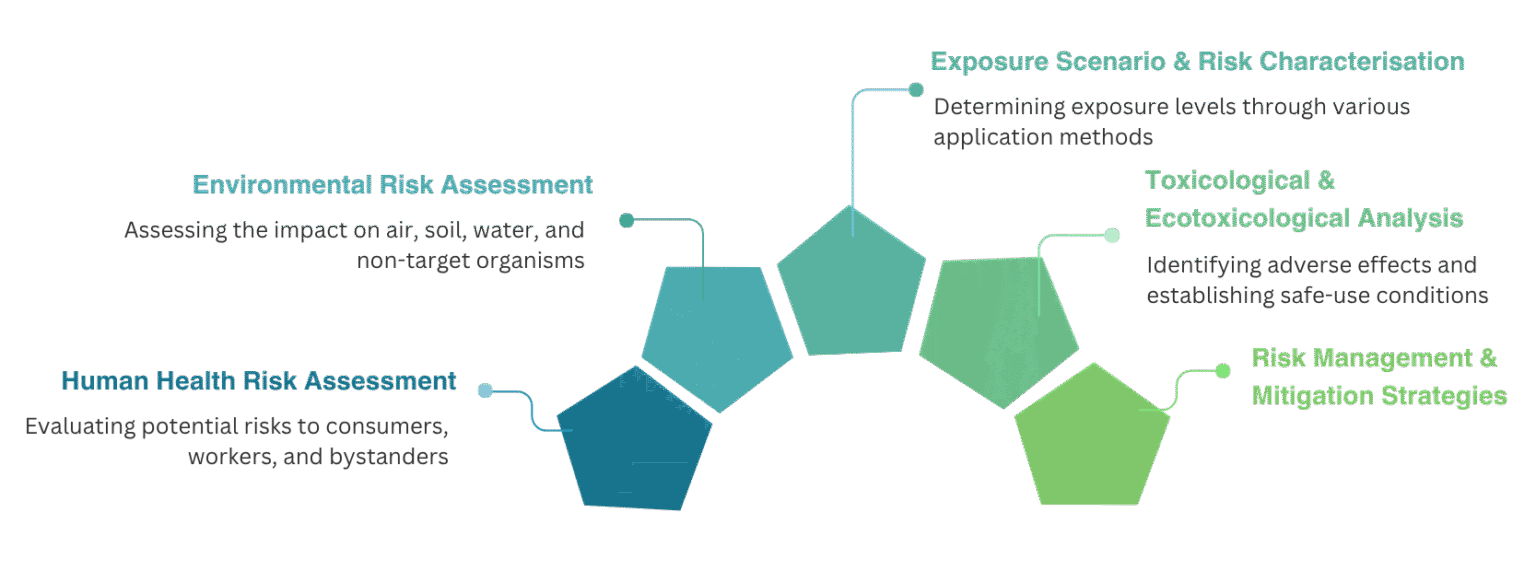
Human Health Risk Assessment
- Toxicological Evaluation – Assessment of acute and chronic toxicity (oral, dermal, inhalation exposure)
- Irritation & Sensitisation Studies – Evaluation of skin/eye irritation, corrosion, and allergic responses
- Reproductive Toxicity Assessment & Mutagenicity & Carcinogenicity Assessment – Identification of long-term health risks
- Dermal Absorption & Exposure Assessment – Determining risk levels for direct contact scenarios
- Acceptable Daily Exposure (ADE) & Threshold Limit Values (TLV) – Establishing safe exposure limits
Environmental Risk Assessment (ERA)
- Ecotoxicological Studies – Evaluating effects on aquatic and terrestrial ecosystems
• Biodegradability & Persistence & Toxicity Testing – Assessing environmental degradation potential
• Soil, Water, and Air Contamination Risk – Identification of environmental hazards
• Environmental Fate & Behaviour Modelling – Predicting product behaviour under various environmental conditions - Risk Mitigation Strategies – Developing control measures to minimise negative impacts
Exposure Scenario & Risk Characterisation
- Occupational Exposure Assessment – Risk evaluation for workers handling the product
• Consumer Exposure Analysis – Risk assessment for end-users and the general population
• Application-Specific Risk Scenarios – Identifying hazards based on intended product use (e.g., disinfectants, insecticides, preservatives)
Risk Management & Mitigation Strategies
- Safety Measures & Precautionary Labelling – Providing accurate hazard communication
• Personal Protective Equipment (PPE) Recommendations – Ensuring proper usage instructions - Product Reformulation Consultation – Advising on changes to reduce toxicity and risk
Regulatory Compliance & Dossier Support
- Risk Assessment Report Preparation – Generating submission-ready reports for BPR
- Data Gap Analysis & Study Recommendations – Identifying missing data and necessary tests
- Submission & Follow-up – Managing risk assessment approvals
Do Your Risk Assessment with Chemleg
With a team of regulatory professionals, Chemleg ensures full compliance with the EU BPR Regulation.
Fill out the form today to benefit from our tailored biocidal product risk assessment services.
Frequently Asked Questions
How many steps does the biocidal product risk assessment process involve?
The risk assessment process typically includes four main steps:
- Hazard Identification
- Dose-Response Assessment
- Exposure Assessment
- Risk Characterisation
What factors are considered in a risk assessment?
Factors include the chemical and toxicological properties of active and co-formulants, the intended use and application method of the product, type of user, and potential exposure routes.
What happens if an unacceptable risk is identified during the risk assessment?
If such a risk is identified, the product may not be placed on the market unless risk mitigation measures are implemented to reduce the risk to an acceptable level.
What documents are required for the risk assessment?
Comprehensive technical documents are required, including physicochemical properties, toxicological and ecotoxicological data, exposure scenarios, and labelling and packaging information.







